EFPIA
This is a placeholder

EFPIA - Benchmarking International Cost-Effectiveness Thresholds: Implications for Biopharmaceutical Innovation
This report examines cost-effectiveness thresholds across 36 countries (the EU-27 and selected high-income international countries), focusing on how health systems apply thresholds to inform healthcare decision-making
Summary
- Out of 36 countries included in the research, the UK is one of only 8 countries (22%) with an explicit cost-effectiveness threshold, while 13 countries including France, Germany, and Spain have no threshold at all
- The UK's threshold of £25,000 per QALY is considerably below the international average of £33,400
- Only 5 countries have lower thresholds than the UK: Greece, Portugal, Slovenia, Norway, and Croatia
- The threshold has not been increased since 1999; if it had been adjusted for inflation, the UK's threshold would be £49,300, almost double its current value
- When considering its economic context, the UK's threshold is more than 30% lower than its GDP per capita, indicating a much more conservative approach compared to other countries
Introduction
The United Kingdom (UK) has used cost-effectiveness (CE) thresholds to guide healthcare funding decisions for over 25 years.[1] The National Institute for Health and Care Excellence (NICE), which sets guidance applicable to England, Wales, and Northern Ireland, has maintained a threshold of £20,000-30,000 per quality-adjusted life year (QALY) since 1999.[2] New research by Charles River Associates, commissioned by EFPIA, has assessed the cost per QALY thresholds across 36 countries (the EU-27 and 9 selected high-income nations).[3] This fact sheet summarises the key findings from the analysis, focusing specifically on how the UK's long-standing threshold compares to current international standards.
Methodology
The research focused on the EU-27 and a selected group of high-income international countries: Australia, Canada, Japan, New Zealand, Norway, South Korea, Switzerland, the United Kingdom (UK), and the United States (US). These countries were chosen because they are often used as reference points in international policy discussions and collectively represent diverse health system structures and decision-making.
The research focused on five parameters: the presence and type of CE thresholds (explicit versus implied), the threshold value in terms of cost per QALY and method of determination, the threshold value relative to GDP per capita, the use of threshold modifiers such as those for severity or rarity, and the application of budget impact thresholds.
Data sources included official health technology assessment (HTA) documents, academic literature, and grey literature. Information was validated with European national trade associations. Where countries had threshold ranges, midpoints were used for comparison. All values were converted to GBP for consistency[4].
Key findings
Presence of CE thresholds
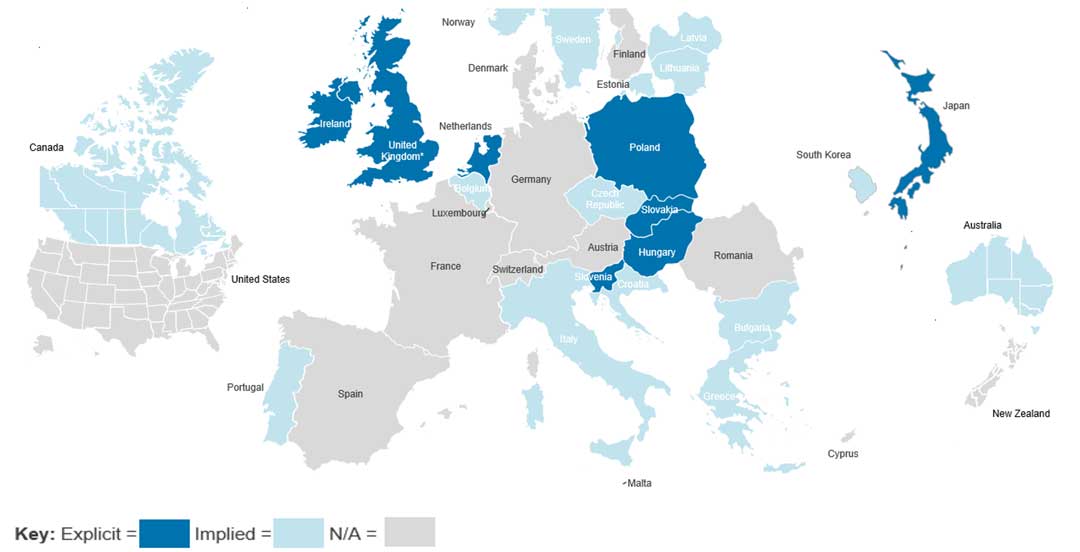
The UK's use of an explicit threshold is relatively uncommon. The research found that of the 36 countries examined, only 8 countries (22%) have explicit thresholds formally defined in legislation or HTA guidelines, with the UK being one of these (see Figure 1. below). Many countries, 15 in total (42%), use implied thresholds i.e. those that can be inferred from past HTA decisions rather than set in law or guidelines.
Perhaps most notably, 13 countries (36%) have no identifiable cost-effectiveness threshold at all. This includes major European economies such as France, Germany and Spain, which either use different assessment approaches or consider cost-effectiveness without applying a fixed threshold.
Figure 1. Map of CE thresholds
The value of the threshold (cost per QALY) & method for determination
Of the countries that do apply CE thresholds, the UK's position is notably low. At £25,000 per QALY (the midpoint of the £20,000-30,000 range), the UK sits well below the international average [5] of £33,400 across all 36 countries. Figure 2 below illustrates this, with countries ordered from highest to lowest threshold.
Figure 2. International cost per QALY thresholds
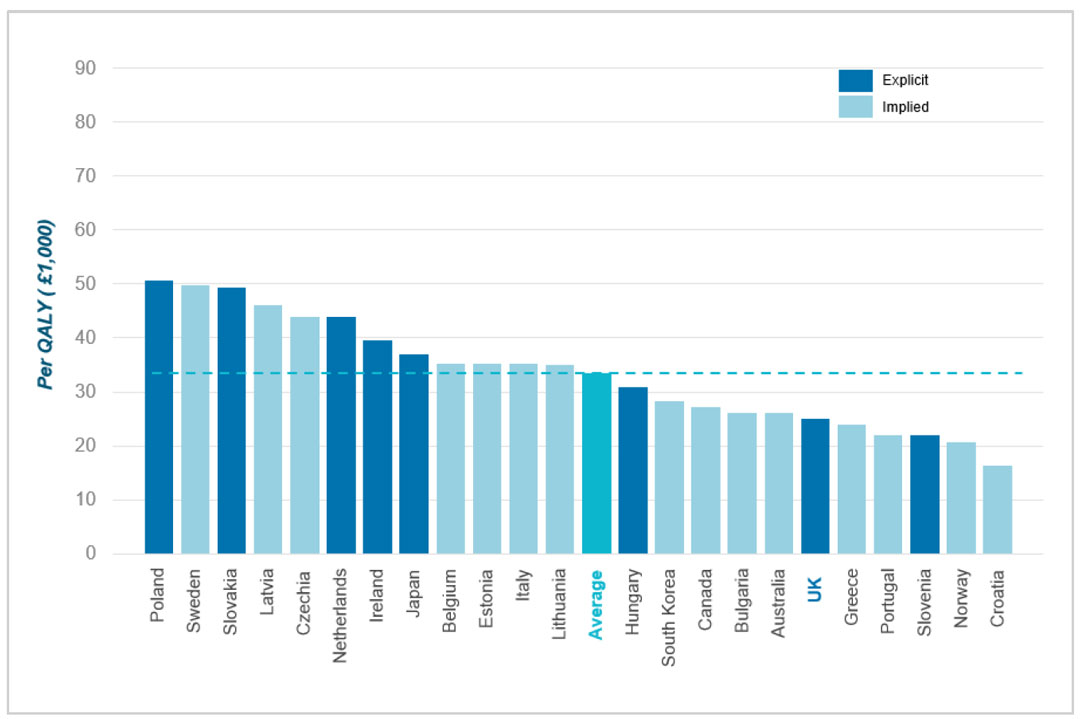
Poland was found to have the highest cost per QALY threshold at £50,700, more than double the UK's threshold. Among countries with explicit thresholds, Poland, and Slovakia (£49,300) have the highest. The UK is in the lower third overall, with only 5 countries having lower thresholds: Croatia, Greece, Norway, Portugal and Slovenia. This positioning suggests that the UK applies considerably more stringent cost-effectiveness requirements than other high-income countries.
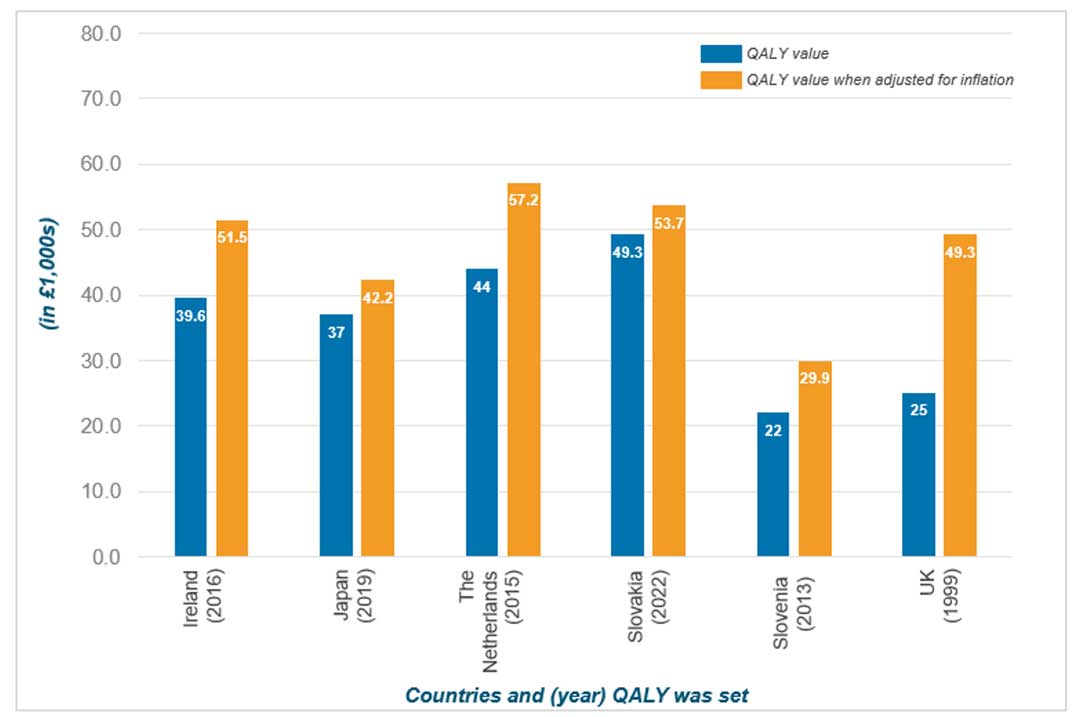
The UK's low threshold has not increased since it was first introduced in 1999. If the UK's threshold had kept pace with inflation over this period, it would now stand at approximately £49,300 per QALY, nearly double its current value (see Figure 3 below).
Figure 3. Cost per QALY threshold vs. value if adjusted for inflation
Cost per QALY thresholds vs GDP per capita
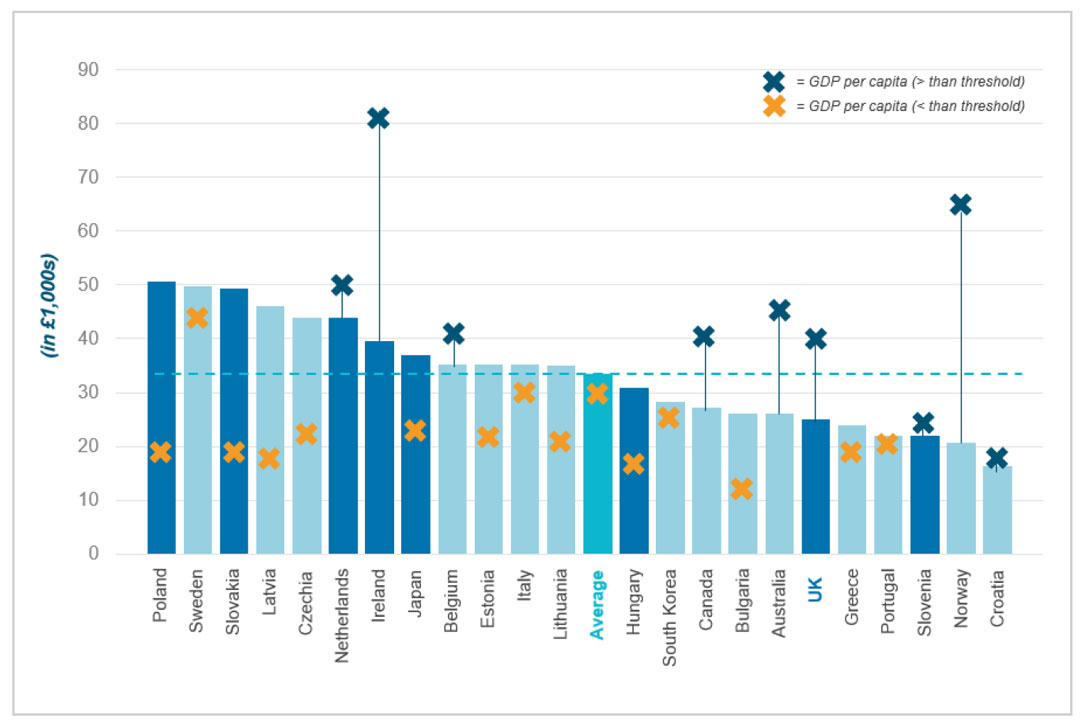
Benchmarking thresholds against GDP per capita provides insight into how countries value health gains relative to their economic capacity. Among the 36 countries assessed, the analysis reveals varying approaches to setting thresholds in relation to national wealth (see Figure 4 below).
The UK is one of several countries where the cost per QALY threshold sits substantially below GDP per capita, indicated by a blue marker above the bar. The difference is particularly significant, with the UK's threshold is more than 30% lower than its GDP per capita. This indicates a comparatively conservative QALY threshold in the UK system, similar to the approach taken by Australia, Canada and Norway.
By contrast, countries like Poland, Sweden and Slovakia have thresholds that exceed their GDP per capita, shown by an orange marker. These countries apply more generous thresholds relative to their economic capacity.
Figure 4. International cost per QALY thresholds vs GDP per capita
The use of modifiers
The UK applies a severity modifier within its HTA framework. NICE's severity modifier allows additional QALY weighting for a small number of very severe diseases (through the HST programme)[6]. This modifier does not change the baseline threshold of £20,000-30,000 per QALY but adjusts the QALY calculation itself. Internationally, 13 countries use formal modifiers including for rarity, severity, and specific therapeutic areas.
Budget impact
The UK also operates a budget impact test alongside its CE threshold. This allows NHS England to seek commercial negotiations or phase rollout for any newly approved drug that is expected to cost over £40 million in any of its first 3 years. Internationally, while 27 of 36 countries consider budget impact in decision-making, only 5 have formal thresholds in place. The UK is among those with a formal threshold, providing an additional mechanism to manage healthcare expenditure beyond cost-effectiveness considerations alone.
Conclusion
The UK is one of only 8 countries with an explicit CE threshold. At £25,000 per QALY, the UK's threshold is considerably below the international average of £33,400 and ranks in the lower third of all countries examined. Major European economies like France, Germany and Spain operate without formal thresholds, potentially offering greater flexibility in funding decisions.
A key issue is that the threshold has not been raised since it was first introduced in 1999. While comparable nations like Ireland, the Netherlands, and Sweden maintain significantly higher thresholds, the UK's has remained frozen for over 25 years. If adjusted for inflation, it would stand at £49,300, nearly double its current value.
In addition, the UK's GDP per capita is more than 30% higher than its threshold, indicating there is potential for the threshold to align more closely with economic capacity. While the UK employs severity modifiers to provide some flexibility, these do not address the underlying issue of the baseline threshold.
References
- McCabe, C., Claxton, K., & Culyer, A. J. (2008). The NICE cost-effectiveness threshold: What it is and what that means. PharmacoEconomics, 26(9), 733-744.
- Scotland uses this range as a guide within a slightly different decision-making framework. Throughout this paper, the UK refers to decisions made by England’s NICE which is applicable to England, Wales and Northern Ireland.
- EFPIA. (2025). Benchmarking International Cost-Effectiveness Thresholds: Implications for Biopharmaceutical Innovation.
- The currency conversion rate used was 1 EUR = 0.88 GBP.
- The average includes all countries with an explicit or implied threshold. Countries with no identified threshold were not included in the calculation.
- Through the Highly Specialised Technologies (HST) programme, the majority of medicines have a £100k per QALY threshold. As such, this figure has been used in the analysis; in extremely rare instances the threshold can go up to £300k per QALY.
